Foot and Mouth Disease: A Highly Contagious Threat to Livestock 🦠🐄
Foot-and-mouth disease (FMD) is an extremely contagious
disease affecting domesticated and wild ungulates. It is characterized by the
presence of vesicles in the mouth and on the feet. Surprisingly, even hedgehogs
and humans can become infected, albeit rarely. 🚫🦔🙅♂️
Aetiology 🧪
FMD is caused by a virus belonging to the aphthovirus genus
in the Picornaviridae family. There are seven distinct types of FMD virus,
namely A, O, C, SAT 1, SAT 2, SAT 3, and Asia 1. Each type encompasses numerous
strains, ranging from closely related ones to those that differ significantly
in their antigenic properties.
Distribution 🌍
FMD is endemic throughout sub-Saharan Africa, extending as
far south as Tanzania. It is also prevalent in Equador, Bolivia, Peru, parts of
Brazil, Columbia, and Venezuela in South America, as well as in most of the
Middle East and Far East. However, countries like Canada, Central and North
America, Australia, New Zealand, Japan, Argentina, Chile, and South Korea
remain free of FMD. Although most of Europe is also free from the disease,
occasional outbreaks occur despite strict import regulations. In Southern
Africa, FMD is primarily confined to wildlife in game parks, with occasional
spillover into neighboring cattle areas.
The most widespread FMD virus types are O and A, prevalent
in South America, the Middle East, and Asia. SAT 1, SAT 2, and SAT 3 are
usually restricted to Africa but have sporadically spread to the Middle East.
Asia 1 occurs in the Far East and India, with occasional incursions into the
Middle East. Type C, on the other hand, rarely causes outbreaks in Asia and has
nearly disappeared.
Epidemiology 🌬️🐮
FMD is highly contagious, and a mere ten infectious units
can initiate the disease in a bovine through the respiratory route. The virus
can survive in dry fecal material for up to 14 days in summer and in slurry for
up to six months in winter. It can persist in urine for 39 days and on soil for
three days in summer and up to 28 days in winter. FMD virus is susceptible to
inactivation by extreme pH levels—below 6.0 and above 10.0—while remaining
stable between pH 7.2 and 7.6. At 4°C, the virus can survive up to a year in
suitable media, but higher temperatures reduce its viability to weeks or even
minutes.
The disease spreads primarily through the movement of
infected animals, with sheep, goats, and wild ungulates playing a significant
role due to their ability to carry the virus with mild or no clinical signs.
Pigs, on the other hand, are highly contagious and can excrete up to 400
million infectious units per day—3000 times more than infected bovines, sheep,
or goats. Infected cattle can also transmit the virus through milk products,
semen, and even before the appearance of clinical signs. Lorries, fomites, and
stockmen can become contaminated with the virus from infected carcasses,
although the acidic conditions following rigor mortis are usually sufficient to
inactivate the virus in meat.
Windborne spread of FMD virus is a significant concern,
particularly in regions with favorable climates for virus survival. Evidence
suggests that the virus can travel over long distances—up to 250km over the sea
and 60km over land. Spread through the wind depends on various factors,
including virus production by infected animals, weather conditions, topography,
and the susceptibility of animals exposed to the airborne virus. Cattle, with
their large respiratory volume, are particularly susceptible to infection
through inhalation of low quantities of the virus, making them highly vulnerable
to airborne transmission.
Cattle that have recovered from FMD or have been vaccinated
can harbor the virus in their pharyngeal region for several months, resulting
in a carrier state. Carrier animals, despite being difficult to transmit the
disease to susceptible animals under experimental conditions, are believed to
have initiated outbreaks based on circumstantial evidence and sequencing of
outbreak strains.
Transmission and Pathogenesis🦠📊
Cattle are most susceptible to FMD through intradermal
inoculation into the tongue. However, natural infection occurs primarily
through inhalation of droplets containing the virus or ingestion of
contaminated materials. Just one infectious unit can cause infection via
intradermolingual inoculation, while inhalation may require 10 to 100
infectious units. Ingestion of the virus typically demands a higher quantity,
although young calves can be infected with lower doses through insufflation of
infected milk.
The primary site of viral replication after inhalation is
the pharynx and lymphoid tissues in the upper respiratory tract. From there,
the FMD virus enters the bloodstream, disseminates throughout the body, and
replicates in other glandular tissues. The virus appears in various body
fluids, including milk, urine, respiratory secretions, and semen, even before
the onset of clinical signs. However, the majority of virus shedding occurs
during the early vesicular stage of the disease. An infected bovine can excrete
large numbers of infectious units, posing a significant risk to uninfected
cattle in the herd and potentially overcoming waning vaccine-induced immunity.
The incubation period for FMD can range from two to 14 days,
depending on factors such as the route of infection, virus dose, strain
virulence, and host susceptibility. When susceptible cattle come into contact
with an infected animal, the incubation period is typically two to four days.
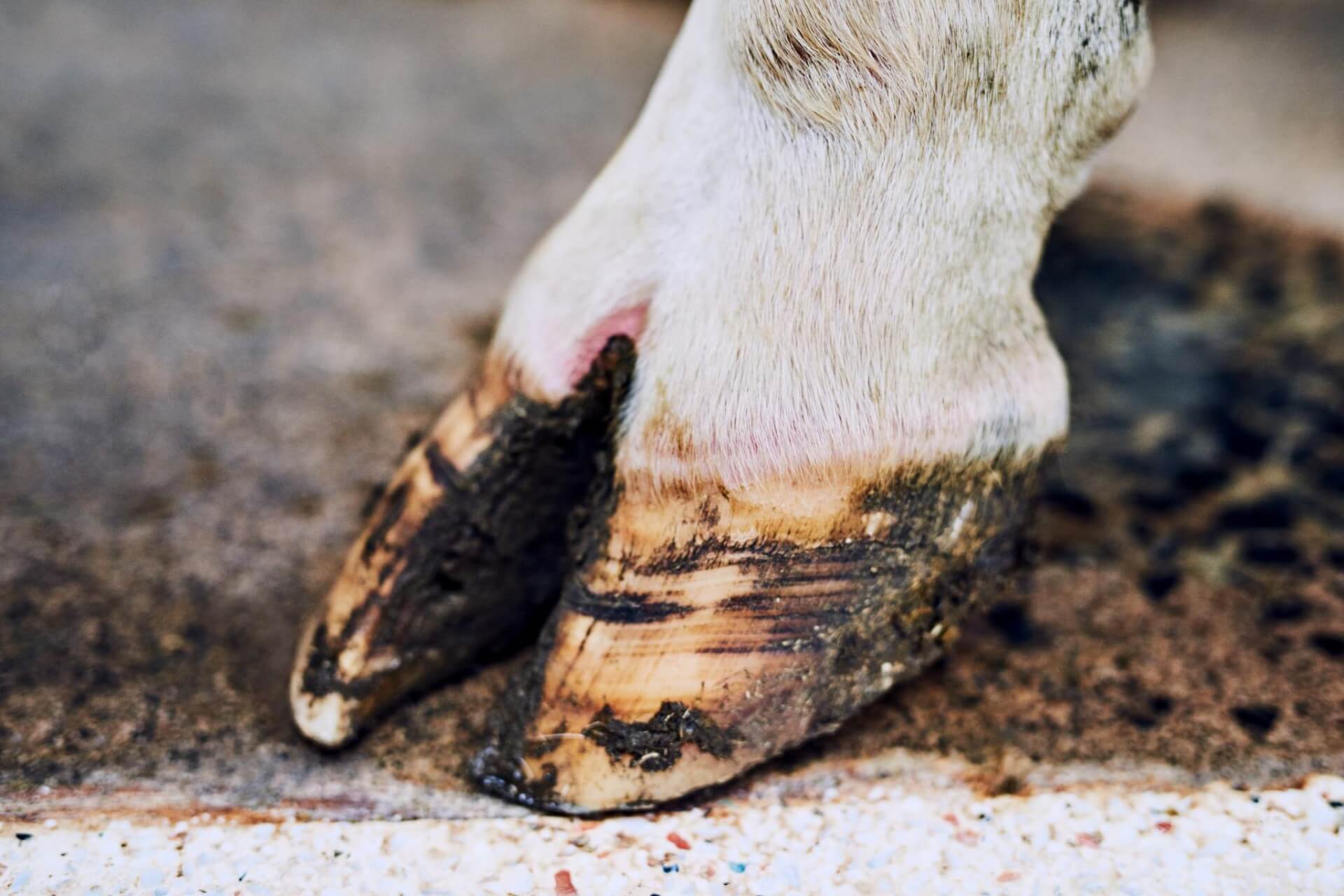
Clinical Signs🤒👅
Following an incubation period of two to 14 days, cattle
infected with FMD exhibit various clinical signs. Initially, they experience
pyrexia (fever) reaching around 40°C (104°F), which lasts for one to two days.
Vesicles then develop on the tongue, hard palate, dental pad, lips, muzzle,
coronary band, and interdigital space. Lactating cows may also have vesicles on
their teats. In young calves, the virus can invade and destroy developing heart
muscle cells, causing mortality before vesicles develop. The vesicles in the
mouth rupture within one to two days, leaving shallow ulcers surrounded by
fragments of epithelium. On the tongue, the vesicles often merge, resulting in
a substantial loss of dorsal epithelium. Vesicles on the feet persist for two
to three days before rupturing, depending on the terrain or flooring in the
cattle's environment.
Mouth lesions typically heal rapidly, filling with fibrin
and transforming into pink, fibrous tissue by the tenth day after vesicle
formation. However, normal tongue papillae do not fully regenerate at this
stage. Foot lesions take longer to heal and are prone to secondary bacterial
infections. Under-run heels may occur due to the initial vesicles and subsequent
bacterial invasion.
Infected cattle salivate excessively, develop nasal
discharge that progresses from mucoid to mucopurulent, and stamp their feet to
alleviate discomfort. They may prefer lying down and resist attempts to stand.
Cattle with teat lesions become difficult to milk, and the damaged teats are
prone to secondary mastitis.
Affected cattle quickly deteriorate, experiencing weight
loss and a dramatic drop in milk production that cannot be recovered during the
remaining lactation period. Some animals never fully regain their previous
condition due to the development of thyroid gland lesions, resulting in a
condition known as "hairy panters."
An outbreak of FMD can have devastating economic
consequences, especially in intensively farmed regions. However, in extensive
husbandry systems found in South America and Africa, where cattle productivity
expectations are low, FMD may seem less significant compared to other prevalent
diseases like clostridial, haemoparasitic, and deficiency diseases. This perspective
can hinder efforts to control FMD effectively or introduce more intensive
farming practices or a dairy industry.
Pathology🧪🔬
During FMD infection, the epithelial cells in the stratum
spinosum of the skin undergo ballooning degeneration, leading to the
development of vesicles and bullae—characteristic features of the disease.
Squamous epithelial cells in the rumen, reticulum, and omasum can also be
affected. In young animals, the virus invades myocardial cells, resulting in
macroscopic grey lesions, particularly in the left ventricular wall, which
gives it a striped appearance, resembling a "tiger heart." Skeletal
muscle cells may also undergo hyaline degeneration.
Diagnosis 👩⚕️🔍
The initial diagnosis of FMD is typically based on clinical
signs and may involve assessing contact between the affected herd and infected
animals or reports of FMD in the vicinity. In fully susceptible herds, the
clinical signs are often severe and pathognomonic. However, in endemic regions
with partial natural or vaccine-induced immunity, clinical signs may be mild
and easily overlooked. It is crucial to investigate all vesicular lesions in
cattle as potential cases of FMD.
Laboratory confirmation of FMD requires submitting adequate
samples under appropriate conditions. A minimum of 2 square cm of epithelium
from a ruptured vesicle, suspended in a mixture of glycerine and buffered
phosphate, should be sent to a designated laboratory equipped to handle FMD
virus and perform type-specific tests.
In countries where FMD is controlled through vaccination,
outbreak strains must be related to existing vaccine strains. This can be
achieved through microneutralization tests using mixtures of field virus and
antisera to a vaccine virus. Serum titers are measured to determine the
antigenic relationship between field and vaccine strains, influencing the
effectiveness of the vaccine in controlling the outbreak.
Various diagnostic techniques are available, including ELISA
to detect virus antigen, virus isolation using cell cultures, PCR for genome
detection, and serological tests such as virus neutralization and ELISA to
measure antibody levels in vaccinated animals or those exposed to FMD.
Control and Economic Impact💰🛡️
Controlling FMD involves preventing virus introduction,
minimizing stock infection, and halting virus spread from infected animals.
Each country adopts control strategies based on economic and practical
considerations.
Quantifying the economic impact of FMD is challenging.
Direct costs, such as vaccination, culling infected animals, movement
restrictions, and market closures, can be measured. However, indirect costs,
like the loss of potential export markets, are more significant yet difficult
to estimate accurately.
Countries striving to prevent or eliminate FMD face two
options: routine vaccination of all cattle (and possibly other livestock) or
refraining from vaccination and relying on slaughter during outbreaks. The most
economical approach depends on critical point analysis or determining the point
at which costs for each policy become equal. Factors to consider include the
cost of vaccines and their administration or storage as a strategic reserve.
Additionally, the cost of controlling an outbreak, including ring vaccination,
slaughtered animals, production loss, and trade interruptions, must be
calculated and multiplied by the estimated number of outbreaks.
Assessing the economic significance of FMD control requires
a well-functioning veterinary infrastructure capable of diagnosing and managing
the disease. Without such support, estimating the true cost of FMD becomes an
academic exercise.
🐄🚫🦠
Protecting Livestock: The Battle Against Foot-and-Mouth Disease 🦠🚫🐄
Prevention and control measures 🛡️🔒
Preventing the introduction of FMD virus is crucial in
controlling the disease. Countries implement strict biosecurity measures at
borders and regulate the movement of animals and animal products to minimize
the risk of virus transmission. Quarantine protocols and monitoring of
livestock farms and markets play a significant role in preventing the spread of
FMD.
Vaccination is another essential tool in controlling FMD.
Vaccines are developed using inactivated or attenuated strains of the virus and
administered to susceptible animals. Vaccination can help reduce the severity
of the disease, limit virus shedding, and protect livestock populations from
outbreaks. However, the effectiveness of vaccination programs depends on
factors such as vaccine quality, coverage, and the matching of vaccine strains
to circulating field strains.
In the event of an outbreak, rapid response is crucial.
Infected animals are typically culled to prevent further spread of the virus.
Surrounding areas may be placed under movement restrictions, and strict
biosecurity measures are enforced to contain the disease. Comprehensive
surveillance, including clinical monitoring and laboratory testing, helps
identify new cases and track the spread of the virus.
International cooperation is vital in combating FMD,
especially in regions where the disease is endemic or where cross-border
movements of animals occur. Collaborative efforts involve sharing information,
harmonizing control strategies, and implementing coordinated vaccination
programs. Organizations such as the World Organisation for Animal Health (OIE)
play a crucial role in facilitating global cooperation and providing guidelines
for FMD control.
Economic impact of FMD💰💔
The economic consequences of FMD can be devastating for
affected countries. The direct costs of control measures, including
vaccination, surveillance, culling, and compensation to farmers, can be
substantial. Additionally, trade restrictions imposed by importing countries
following an outbreak can result in significant losses for livestock producers
and related industries. The negative impact on export markets, tourism, and
overall economic stability can be long-lasting.
In regions heavily reliant on livestock production, such as
rural communities, the socioeconomic consequences of FMD outbreaks can be
particularly severe. Farmers may suffer substantial financial losses, and the
loss of productive animals can disrupt the local economy. Employment
opportunities, market access, and food security can be jeopardized, affecting
the livelihoods of numerous individuals and communities.
Efforts to control and eradicate FMD require substantial
investment in veterinary infrastructure, research and development, surveillance
systems, and public awareness campaigns. Governments and international
organizations must allocate resources to support these initiatives and
strengthen veterinary services to effectively combat the disease.